In 2006, soon after she launched her own laboratory, neuroscientist Jane Foster discovered something she felt sure would set her field abuzz. She and her team were working with two groups of mice: one with a healthy selection of microorganisms in their guts, and one that lacked a microbiome. They noticed that the mice without gut bacteria seemed less anxious than their healthy equivalents. When placed in a maze with some open paths and some walled-in ones, they preferred the exposed paths. The bacteria in the gut seemed to be influencing their brain and behaviour.
Foster, at McMaster University in Toronto, Canada, wrote up the study and submitted it for publication. It was rejected. She rewrote it and sent it out again. Rejected. “People didn’t buy it. They thought it was an artefact,” she says. Finally, after three years and seven submissions, she got an acceptance letter1.
John Cryan, a neuroscientist at University College Cork in Ireland, joined the field about the same time as Foster did, and knows exactly how she felt. When he began talking about the connections between bacteria living in the gut and the brain, “I felt very evangelical”, he says. He recalls one Alzheimer’s disease conference at which he presented in 2014. “I’ve never given a talk in a room where there was less interest.”
Today, however, the gut–brain axis is a feature at major neuroscience meetings, and Cryan says he is no longer “this crazy guy from Ireland”. Thousands of publications over the past decade have revealed that the trillions of bacteria in the gut could have profound effects on the brain, and might be tied to a whole host of disorders. Funders such as the US National Institutes of Health are investing millions of dollars in exploring the connection.
But along with that explosion of interest has come hype. Some gut–brain researchers claim or imply causal relationships when many studies show only correlations, and shaky ones at that, says Maureen O’Malley, a philosopher at the University of Sydney in Australia who studies the field of microbiome research. “Have you found an actual cause, or have you found just another effect?”
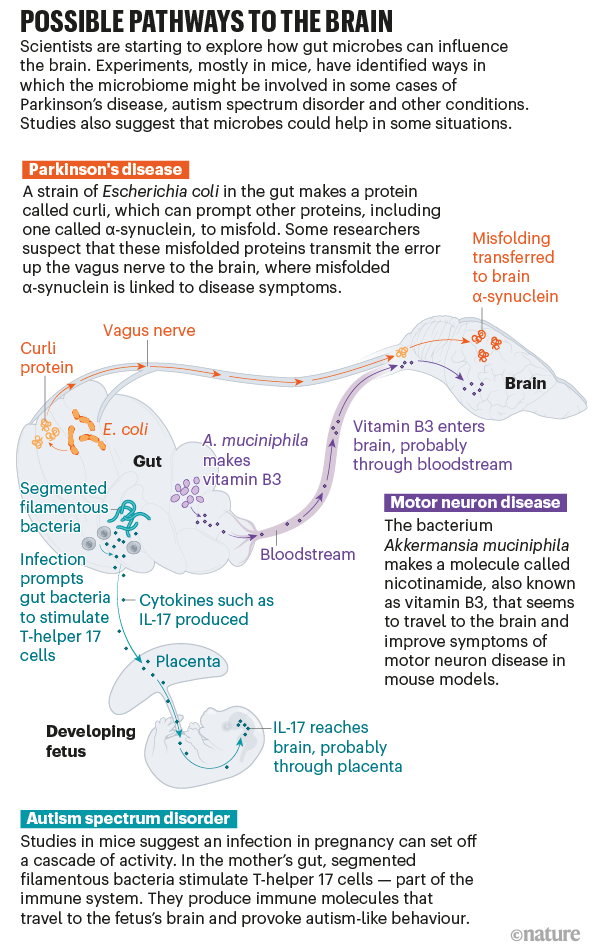
In recent years, however, the field has made significant strides, O’Malley says. Rather than talking about the microbiome as a whole, some research teams have begun drilling down to identify specific microbes, mapping out the complex and sometimes surprising pathways that connect them to the brain. “That is what allows causal attributions to be made,” she says. Studies in mice — and preliminary work in humans — suggest that microbes can trigger or alter the course of conditions such as Parkinson’s disease, autism spectrum disorder and more (see ‘Possible pathways to the brain’). Therapies aimed at tweaking the microbiome could help to prevent or treat these diseases, an idea that some researchers and companies are already testing in human clinical trials.
It is early days, but the prospect of new therapies for some of these intractable brain diseases is exciting, says Sarkis Mazmanian, a microbiologist at the California Institute of Technology in Pasadena — particularly given how much easier it is to manipulate the gut than the brain. Getting therapies into the brain has been a long-standing challenge, he says, “but you can sure as hell change the microbiome”.
Tangle transmission
In 1817, the English surgeon James Parkinson described some of the first cases of the “shaking palsy” that would come to be known as Parkinson’s disease. One individual had developed numbness and prickling sensations in both arms. Parkinson noticed that the man’s abdomen seemed to contain “considerable accumulation”. He dosed the man with a laxative, and ten days later his bowels were empty and his symptoms were gone.
Parkinson might have been on to something. Some people who develop the disease experience constipation long before they develop mobility problems. And many researchers have embraced the idea that the disease begins in the gut, at least in some cases.
To understand the idea, it’s useful to know a little about the disease. The hallmark symptoms of Parkinson’s — tremors, stiffness and slowness of movement — appear as the neurons responsible for coordinating motion begin to die. Why these neurons die isn’t fully understood, but a protein known as α-synuclein seems to have a key role. In people with Parkinson’s disease, the protein misfolds. The first misfolded protein causes more to misfold, until harmful clumps known as Lewy bodies begin to form in the brain.
What triggers this cascade? In 2015, Robert Friedland, a neurologist at the University of Louisville in Kentucky, proposed a new theory. He had read that gut bacteria can produce proteins that have a similar structure to the misshapen α-synuclein proteins, so he posited that bacterial proteins might be providing a template for misfolding2. And when he and his colleagues fed rats a particular strain of Escherichia coli that produces one of these clumping proteins, called curli, in the gut, they saw more α-synuclein accumulation in the animals’ brains3. Work published last year by Mazmanian and his team supports Friedland’s theory4.
It’s not yet clear how that signal in the gut reaches the brain, but one likely conduit is the vagus nerve. The vagus connects the brainstem to many organs, including the colon, making it the longest of the twelve cranial nerves that carry signals between the brain and the rest of the body. “It’s really a highway,” Cryan says. And research in humans and animals suggests that it has a crucial role in ferrying at least some messages between the gut and the brain.
In the 1970s, a common therapy for stomach ulcers was to remove all or part of the nerve to curb acid production in the stomach. But in recent decades, researchers noticed a strange side effect: people who had undergone this procedure seemed to be less susceptible to Parkinson’s disease5.
In a study in mice, injecting misfolded α-synuclein into the gut produced it in the brain. But if researchers first removed the vagus nerve, no α-synuclein appeared in the brain6. The injected α-synuclein itself seems to stay in the gut, but Valina Dawson, a neuroscientist at Johns Hopkins University in Baltimore, Maryland, who was an author on the study, thinks that there might be a domino effect: misfolded proteins transmit the error up the vagus nerve until proteins in the brain eventually misfold. Mazmanian and his colleagues are now conducting experiments to see whether the curli protein in the gut can still prompt Parkinson’s symptoms in mice that have had the vagus nerve severed.
Because misfolded proteins are a hallmark of several other conditions that affect the brain, including Alzheimer’s disease and motor neuron disease (amyotrophic lateral sclerosis, or ALS), Friedland says that bacterial proteins could be implicated in these diseases, too. Dawson finds the idea plausible, but says that bacterial amyloids aren’t the only factor to consider. Parkinson’s, for example, is a complex disease that presents differently in different people. Still, she says, “this could be one way to get things started.”
Hastening decline
Proponents of the gut–brain link say that the microbiome could do more than just trigger some cases of neurodegenerative disease: it could also have effects on its severity. Eran Elinav, an immunologist at the Weizmann Institute of Science in Rehovot, Israel, and at the German Cancer Research Center in Heidelberg, was struck by the differences in how ALS can develop: some people with the disease progress slowly, and others deteriorate rapidly. Elinav wondered whether the microbiome helps to explain those differences, so he and his team began working with one of the most common ALS mouse models. When they wiped out the microbiome with antibiotics, or used mice that lacked a microbiome from birth, they saw a much more rapid progression of the disease than in mice with a normal microbiome7.
The team compared gut bacteria in ALS mice with those of their healthy littermates, and found several microbial species that seemed to be linked to disease. They painstakingly transplanted those species, one by one, into another group of mice without any gut bacteria, identifying two species that made ALS symptoms worse, and one that seemed to make them better. “And then we asked ourselves, ‘how does this strain that only lives in the gut impact so amazingly a disease which is focused at the brain?’” Elinav says.
The culprits could be bacterial metabolites — small molecules produced by bacteria that can enter the bloodstream and travel around the body. At least half of all small molecules in the blood are “either made by microbes or modulated by microbes”, Elinav says. He and his team analysed the metabolites produced by the beneficial microbe, and administered one, a molecule called nicotinamide — also known as vitamin B3 — to mice prone to ALS. They found that the molecule entered the brain and improved their symptoms7. “We could prove that there is a bacterium, we could prove that there is a product of the bacterium, and we could prove that it was swimming to the right target organ and doing something favourable to disease course,” he says.
When they compared the microbiomes of people with ALS and those of their unaffected family members, they saw less nicotinamide in the individuals with ALS7. The metabolite is readily available as a supplement, and Elinav says he and his colleagues are planning a clinical trial with it.
At least one group has already tested vitamin B3 as a treatment for ALS in a small trial, albeit a version combined with another compound. They administered it to participants with ALS for four months. Those in the treatment group showed some improvement, but nearly all of the people in the placebo group declined in health8.
“This is just the beginning,” Elinav says. Many more bacteria and metabolites exist, and every cell in the body is open to their effects. Once you realize that, he says, “you start understanding that the effect of the microbiome could expand well beyond where the microbes actually live”.
Generational effects
The effect could even pass from one generation to the next. Take autism spectrum disorder (ASD). The causes are still poorly understood, but infections in a mother during pregnancy seem to increase the risk of ASD in her child, according to epidemiological studies. For example, in a Swedish cohort of nearly 1.8 million people, those whose mothers had been hospitalized for any infection during pregnancy had a 79% higher risk of being diagnosed with ASD9.
Research in mice also supports the link. To mimic an infection, researchers inject pregnant mice with double-stranded RNA, which the body sees as a viral invader. Their pups exhibit more repetitive behaviours and anxiety than do those born to mothers who weren’t injected, and interact less with other mice — symptoms that mirror those of people with ASD10.
Gloria Choi, a neuroscientist at the Massachusetts Institute of Technology’s Picower Institute for Learning and Memory in Cambridge, and her husband and collaborator Jun Huh, an immunologist at Harvard Medical School in Boston, wanted to know why. They zeroed in on a type of cell that defends against bacteria and fungi by producing molecules called cytokines. When Choi and Huh mimicked an infection in their mice, these cells, called T-helper 17 cells, became hyperactive, churning out a particular type of cytokine called IL-17. This molecule travelled into the brains of the developing pups, probably through the placenta, and then bound to brain receptors. This seemed to have a profound effect on the animals: the researchers found that the adult offspring showed increased neural activity, which caused their autism-like behaviours11.
But “not every pregnant woman who is infected or hospitalized during pregnancy necessarily has children with neurodevelopmental disorders or autism”, Huh says. There must be something tipping the mother’s immune system towards this overactive state. Choi and Huh focused on a collection of long, thin gut microbes known as segmented filamentous bacteria, which had previously been shown to promote the formation of T-helper 17 cells. When they treated pregnant mice with an antibiotic to kill these bacteria and then stimulated an immune response, the pups didn’t develop any behavioural differences12.
Choi and Huh, keen to know whether the coronavirus pandemic could lead to an increased risk of ASD, are collecting samples from pregnant women who are infected with SARS-CoV-2 and cataloguing the bacteria in their guts and levels of IL-17 in their blood. Given that the coronavirus, like any other infection, activates the mother’s immune system , it is plausible that SARS-CoV-2 could increase the risk of altered brain development and potentially psychiatric disorders, says David Amaral, who studies ASD at the University of California, Davis. Researchers have not yet found evidence in support of this theory.
Mauro Costa-Mattioli, a neurobiologist at Baylor College of Medicine in Houston, Texas, is also studying the relationship between bacteria and ASD. But rather than looking at microbes that cause the disorder, he has found one that might treat its symptoms.
Costa-Mattioli stumbled on this bacterium by accident about five years ago. At the time, he was working on mice with offspring that have autism-like symptoms. When those mice were housed with neurotypical ones (and ate their excrement, as all mice are wont to do), their ASD-like behaviours disappeared. Costa-Mattioli and his colleagues worked out that the affected mice were missing a particular species of bacterium: Lactobacillus reuteri.
They tested L. reuteri in several other mouse models, and the bacterium was able to reverse some of the ASD-like behaviours in every one. And, just as with the Parkinson’s work, the researchers could block the effect in mice if they severed the vagus nerve13.
Exactly what type of signal L. reuteri sends isn’t yet known. The team has found that some strains of L. reuteri can reverse the behaviours while others cannot, and the researchers are now working to discover which of its genes are involved. If they find the gene that produces a key metabolite, “we can just put it in any bacteria and now we may have a potential treatment”, Costa-Mattioli says. That strategy has yet to be tested.
One group in Italy is already trying L. reuteri as a therapy in 80 children with ASD. Participants will take L. reuteri or a placebo tablet for six months, and have their symptoms monitored. Costa-Mattioli is hoping to launch his own trial soon.
Whether it will work remains to be seen, but Kevin Mitchell, a neurogeneticist at Trinity College Dublin, doesn’t yet find the mouse studies persuasive. And he sees the discussion of therapeutic potential as premature and “a bit irresponsible”, he says, given the complexity of the condition.
Meanwhile, researchers are exploring more brain maladies, including Alzheimer’s disease and depression. Gut microbes might even influence how the brain recovers after injury. Corinne Benakis, a neurobiologist at the Institute for Stroke and Dementia Research at Ludwig Maximilians University of Munich in Germany, and her colleagues treated mice with antibiotics to wipe out some of their gut bacteria before inducing a stroke. They found that antibiotics could reduce the severity of the brain damage14.
In each of these diseases, many mechanistic questions remain. Researchers in the field acknowledge that they have yet to flesh out the pathways from microbe to brain. And the trickiest step will be validating these animal findings in humans and moving into trials. “These are extraordinary claims, which should require extraordinary evidence,” says Mitchell.
But there’s also enormous interest — and not just from academics. In February 2019, Axial Therapeutics in Waltham, Massachusetts, a company co-founded by Mazmanian to develop therapies for neurodegenerative and neuropsychiatric diseases, raised US$25 million in financing. Another company, Finch Therapeutics in Somerville, Massachusetts, which is developing an oral microbiome drug for ASD, announced in September that it had raised $90 million.
Cryan has watched the data pile up since his talk on the subject was met with stony silence. He finds the mounting evidence convincing, and sees enormous promise in microbiome-based therapies. “Unlike your genome, which you can’t do much about except blame your parents and grandparents, your microbiome is potentially modifiable. And that gives great agency to patients,” he says. “That’s really exciting.”
"how" - Google News
February 03, 2021 at 06:12PM
https://ift.tt/36Gdj1U
How gut microbes could drive brain disorders - Nature.com
"how" - Google News
https://ift.tt/2MfXd3I
https://ift.tt/3d8uZUG
Bagikan Berita Ini














0 Response to "How gut microbes could drive brain disorders - Nature.com"
Post a Comment